Abstract
Introduction
Chronic myeloid leukemia (CML) is characterized by the presence of Philadelphia chromosome (Ph). Acquired somatic mutations of JAK2, MPL and CALR are defining pathogenetic events in most cases of classical Ph-negative myeloproliferative neoplasms (MPN). Although chromosomal abnormalities observed in Ph-negative MPNs are not specific, chromosomal analysis is still part of the diagnostic work-up given their potential role in disease progression. The prognostic relevance of i(17q) has been well recognized in acute myeloid leukemia and myelodysplastic syndrome. However, other than some sporadic case reports, its role in MPNs remains unclear.
Method
Cytogenetic data of adult patients diagnosed with CML or classical Ph-negative MPNs from year 1998 through 2016 were reviewed for the presence of i(17q) and its impact on disease progression and patient outcome. Blast phase (BP) was defined as blast percentage in peripheral blood or bone marrow exceeding 20%.
Results
A total of 88 patients, including 71 patients with CML and 17 patients with classical Ph-negative MPNs were identified to have i(17q). In the 17 patients with Ph- MPN, 15 patients had primary myelofibrosis, 1 had polycythemia vera, 1 had essential thrombocytosis.
The median age at diagnosis is 48 years for CML patients and 61 for Ph- MPN patients. i(17q) was present at initial diagnosis in 33 patients, including 26 with CML and 7 with Ph- MPN. In the remaining 55 patients, i(17q) emerged after a median follow-up time of 36.9 months in CML patients and 18 months in Ph- MPN patients. At the emergence of i(17q), 33 patients were in BP, including 27 patients with CML and 6 patients with Ph- MPN. In the remaining 55 patients, 20 patients including 16 with CML and 4 with Ph- MPN, developed BP after a median follow-up time of 8 months (range 0.7 to 72.9 months). Overall, in the 43 CML patients with BP, 38 had myeloid BP, 4 had lymphoid BP, and 1 had mixed phenotype BP. In 10 Ph- MPN patients with BP, all had myeloid BP.
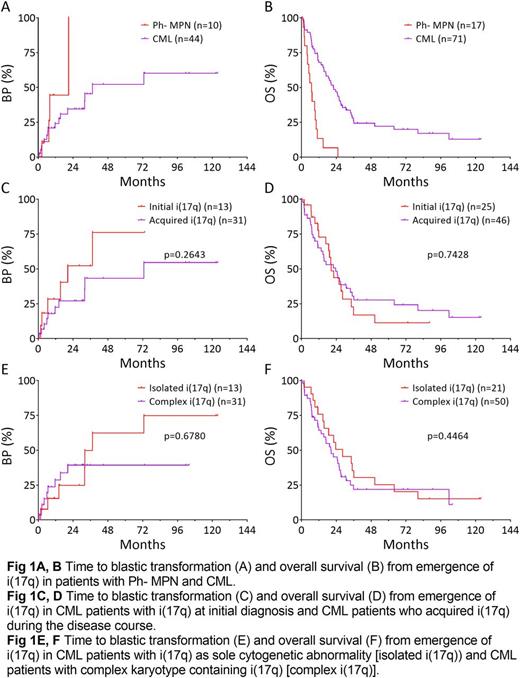
Overall, the emergence of i(17q) was associated with unfavorable outcome. The one-year probability of blastic transformation was 44.4% in patients with Ph- MPN, and 24.1% in those with CML (Fig 1A). The median survival from emergence of i(17q) was 6.9 months in patients with Ph- MPN and 22.3 months in those with CML (Fig 1B). Patients with i(17q) at initial presentation and patients who acquired i(17q) later in disease course had similar risk of progression (Fig 1C) and overall survival (Fig 1D). Patients with isolated i(17q) and patients with complex karyotype containing i(17q) had similar risk of progression (Fig 1E, p=0.6780) and overall survival (Fig 1F, p=0.4464).
Conclusion
i(17q) is associated with rapid progression and unfavorable overall survival in both CML and Ph- MPN. CML with i(17q) tends to progress to myeloid BP. The prognostic impact of i(17q) is not affected by the emergence time nor concurrent cytogenetic abnormalities.
Verstovsek: Incyte: Research Funding; Seattle Genetics: Research Funding; CTI BioPharma Corp: Research Funding; Lilly Oncology: Research Funding; Gilead: Research Funding; NS Pharma: Research Funding; Galena BioPharma: Research Funding; Genentech: Research Funding; Promedior: Research Funding; Blueprint Medicines Corp: Research Funding; Bristol Myers Squibb: Research Funding; Roche: Research Funding; Incyte: Research Funding; NS Pharma: Research Funding; CTI BioPharma Corp: Research Funding; Astrazeneca: Research Funding; Promedior: Research Funding; Gilead: Research Funding; Bristol Myers Squibb: Research Funding; Blueprint Medicines Corp: Research Funding; Celgene: Research Funding; Pfizer: Research Funding; Lilly Oncology: Research Funding; Celgene: Research Funding; Astrazeneca: Research Funding; Genentech: Research Funding; Pfizer: Research Funding; Galena BioPharma: Research Funding; Roche: Research Funding; Seattle Genetics: Research Funding. Cortes: ARIAD: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; Teva: Research Funding; BMS: Consultancy, Research Funding; Sun Pharma: Research Funding; Pfizer: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal